
To get the mass of the precipitate, use its molar mass This means that the reaction will produce Use the molarity and volume of the silver fluoride solution to determine how many moles take part in the reaction a AgCl + b H 2 SO 4 c Ag 2 SO 4 + d HCl Create a System of Equations Create an equation for each element (Ag, Cl, H, S, O) where each term represents the number of atoms of the element in each reactant or product.
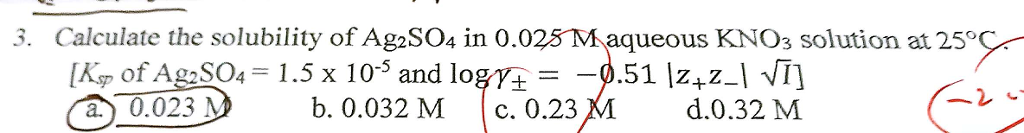
The #color(red)(2):1# mole ratio that exists between silver fluoride and silver sulfate tells you that, regardless of how many moles of the former react, the reaction will produce half as many moles of the latter. Since sodium sulfate is in excess, all the moles of silver fluoride will react. Write a balanced molecular equation, complete ionic equation, and net ionic equation for metathesis reaction. Systematically combine solutions and identify the reactions that form precipitates and gases. Hoh Aqua HO Oh2 Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. Chemistry Chemistry questions and answers 2 AgNO3 (aq) + K2SO4 (aq) 2 KNO3 (aq) + Ag2SO4 (s) The spectator ions in the reaction shown are This problem has been solved You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Purpose Identify the ions present in various aqueous solutions.
#Ag 2so 4 how to#
Silver(I) Sulfide Ag2S Molar Mass Ag2S Oxidation Number. Ag2SO4 Ag2 + SO4 - Chemical Equation Balancer Balanced Chemical Equation Reaction Information Disclaimer Instructions Examples How To Balance Equations. #color(red)(2)AgF_((aq)) + Na_2SO_(4(aq)) -> Ag_2SO_(4(s)) darr + 2NaF_((aq))# Double Displacement (Metathesis) Reactants. What is the molar solubility of silver sulfate (Ag2SO4) in water The solubility-product constant for Ag2SO4 is 1.5 times 10-5 at 25 degree C.

The double replacement reaction that takes place between these two compounds will produce silver sulfate, #Ag_2SO_4#, an insoluble compound, and sodium fluoride, #NaF#, according to the balanced chemical equation So, you're two solutions that contain soluble compounds - silver fluoride, #AgF#, and sodium sulfate, #Na_2SO_4#.


 0 kommentar(er)
0 kommentar(er)
